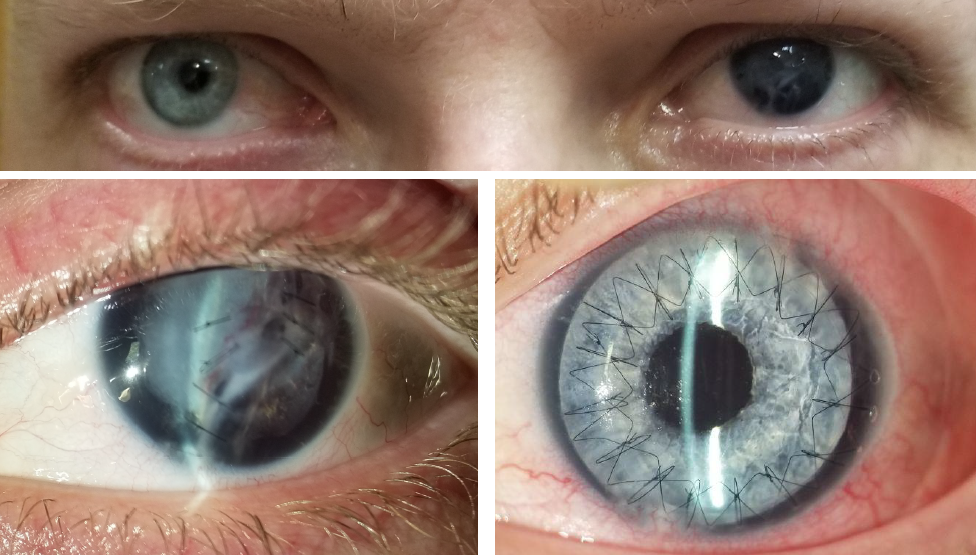
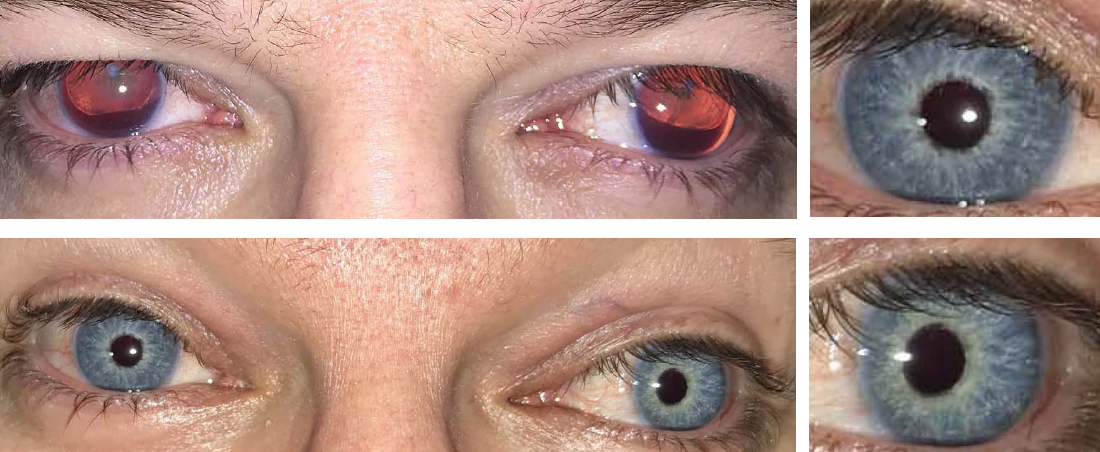
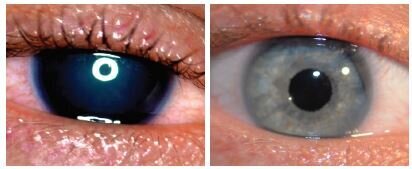
Managing congenital aniridia and acquired iris defects — including, but not limited to, traumatic iris defects and traumatic mydriasis — is often challenging. When iris reconstruction is necessary, the CUSTOMFLEX® ARTIFICIALIRIS is a unique device that offers both surgeons and patients important benefits.
Other Resources
Documents
INDICATIONS
The CUSTOMFLEX® ARTIFICIALIRIS is intended for use as an iris prosthesis for the treatment of iris defects. The CUSTOMFLEX® ARTIFICIALIRIS is indicated for use in children and adults for the treatment of full or partial aniridia resulting from congenital aniridia, acquired defects, or other conditions associated with full or partial aniridia.
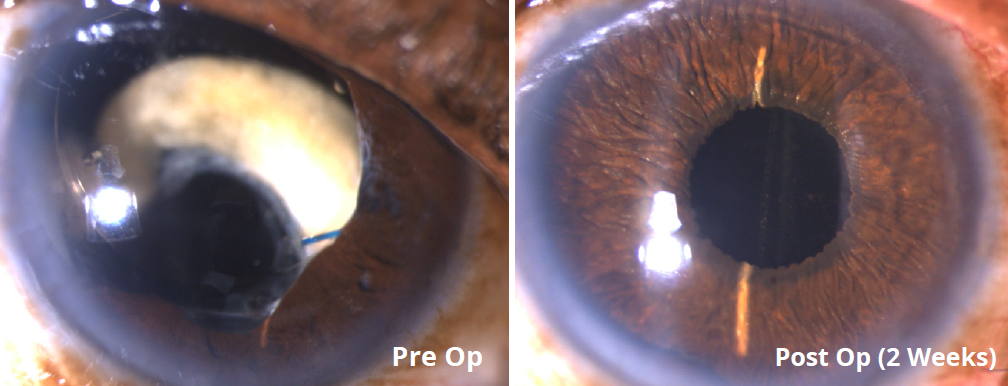
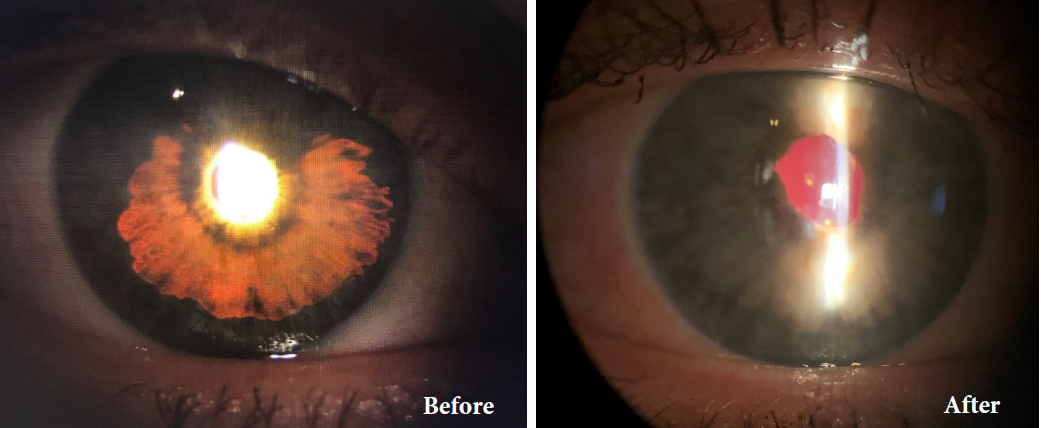
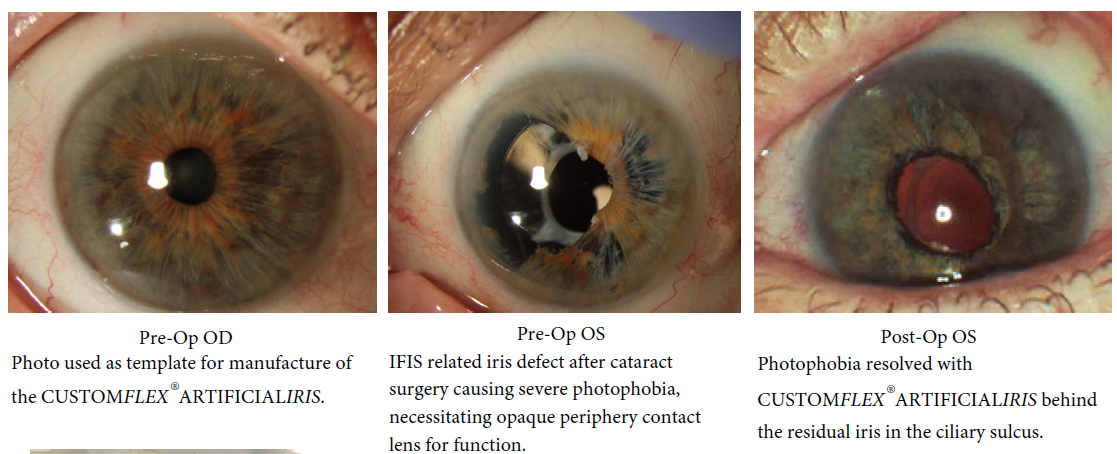
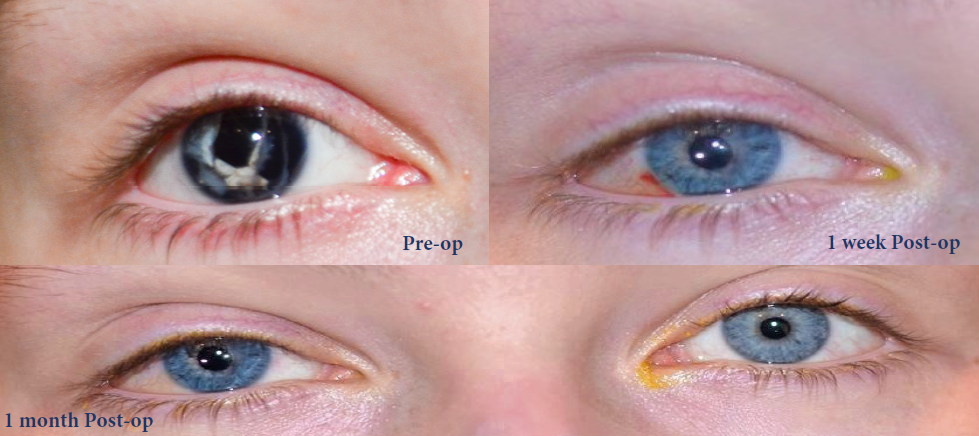
Traumatic Injury
Congenital Aniridia
Oculocutaneous Albinism
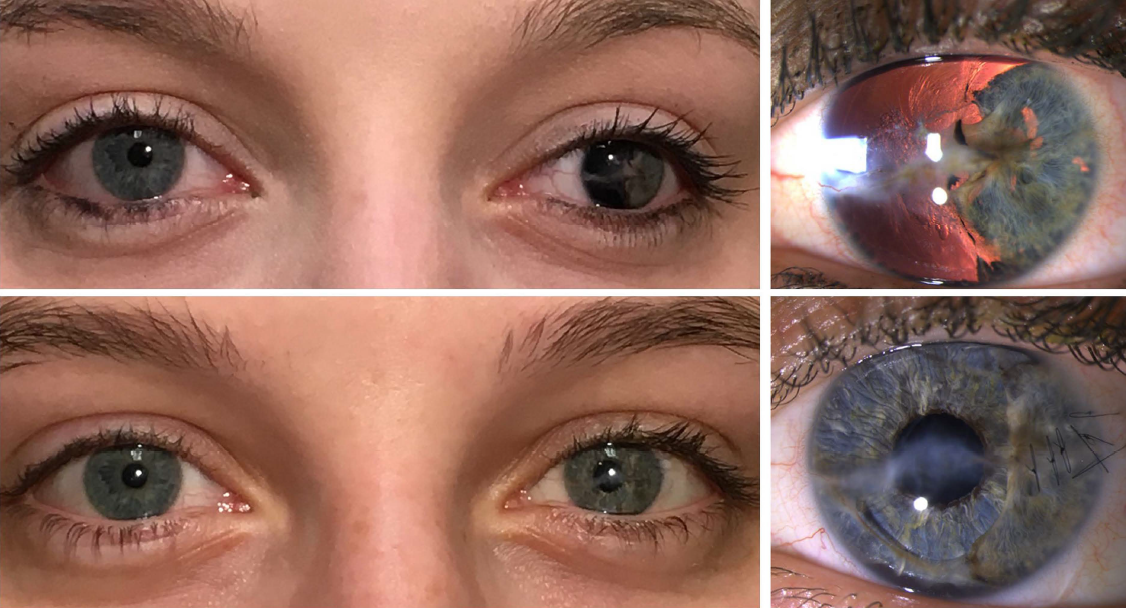
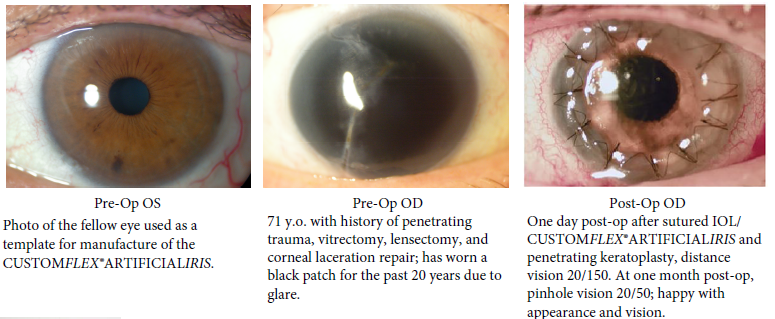
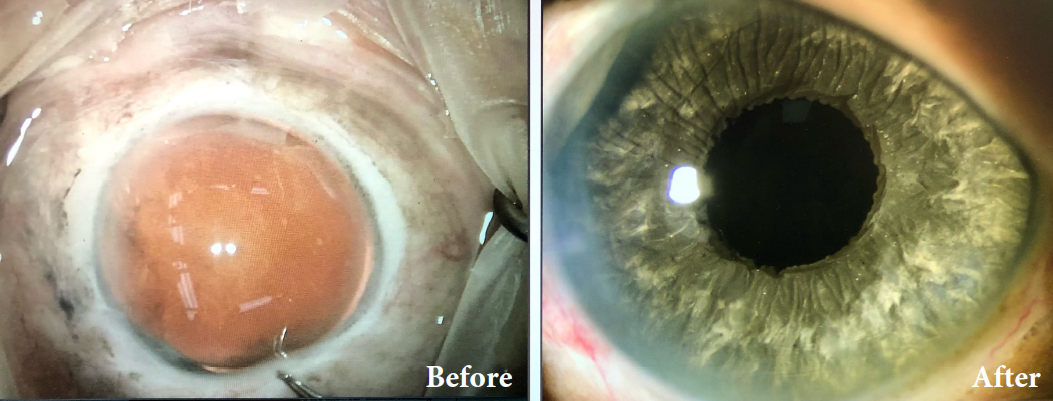
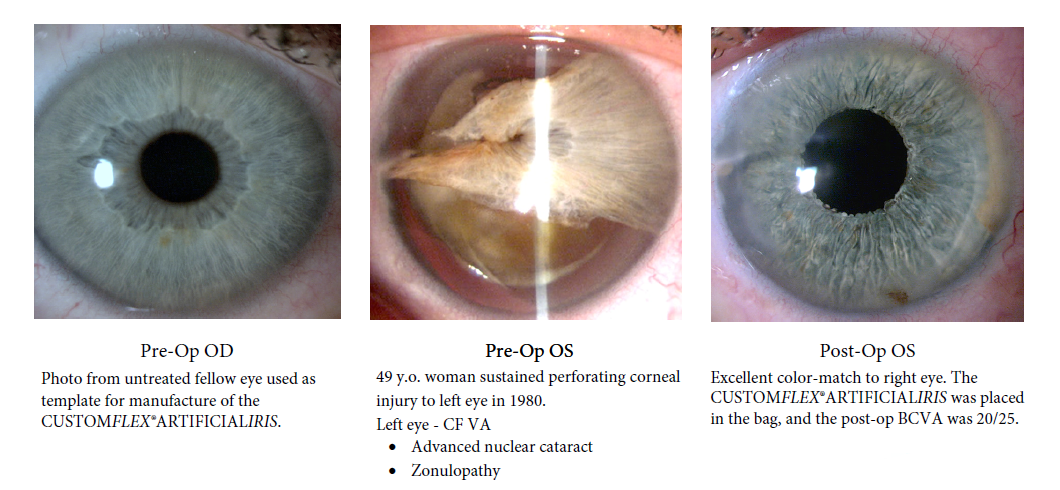
BEFORE AND AFTER IMPLANTATION OF THE CUSTOMFLEX® ARTIFICIALIRIS.
CUSTOM-COLOR MATCH
Every CUSTOMFLEX® ARTIFICIALIRIS is custom-made to match the patient's natural iris tissue, addressing both symptomatic and cosmetic aspects of iris defects.
BENEFITS
Biocompatibility: Long, safe history of highly biocompatible medical-grade silicone
Flexibility: Flexible material that can be folded for insertion through small incisions using forceps or an auto injector
Versatility:
Sutureless and Sutured Surgical Procedures:
Capsular bag placement
Passive sulcus fixation with or without sutures
Suture fixation to the scleral wall
PCIOL Sutured Ex vivo to the CUSTOMFLEX® ARTIFICIALIRIS
Can be easily sized for each patient’s needs
Can be implanted with most intraocular lenses
Limits Light Transmission: Reduces photosensitivity symptoms and can be used to treat transillumination defects with a fixed aperture of 3.35mm and opaque black posterior surface to absorb light
Customized: Every CUSTOMFLEX® ARTIFICIALIRIS is custom-made to match the patient’s natural iris tissue, addressing both symptomatic and cosmetic aspects of iris defects.
PRODUCT SPECIFICATIONS
Overall Diameter: 12.8 mm
Pupil Size: 3.35 mm
Injectable
RECOMMENDED INSTRUMENTATION AND SUPPLIES
CUSTOMFLEX® ARTIFICIALIRIS CLINICAL SCIENCE
The safety and efficacy of the CUSTOMFLEX® ARTIFICIALIRIS was demonstrated in a non-randomized clinical trial of 447 eyes of 389 adult and pediatric patients with aniridia or other iris defects. There was an overall significant improvement in photosensitivity symptoms, quality of life, and vision in pediatric and adult subjects. At study conclusion, 63% of eyes reported no or mild daytime light sensitivity; and, 79% reported no or mild nighttime light sensitivity, while more than 70% of eyes reported no or mild glare during the day or at night. 94% of subjects were satisfied with the CUSTOMFLEX® ARTIFICIALIRIS appearance.
PRODUCT SAFETY INFORMATION
Contraindications
Contraindications: CUSTOMFLEX® ARTIFICIALIRIS is contraindicated for patients with certain eye conditions, such as uncontrolled inflammation, severe chronic uveitis, microphthalmus, untreated retinal detachment, untreated chronic glaucoma, rubella cataract, rubeosis of the iris, proliferative diabetic retinopathy, Stargardt’s retinopathy, or intraocular infections, or in pregnant women.
Warnings
Implantation of the CUSTOMFLEX® ARTIFICIALIRIS is not recommended in children who are less than 3 years of age because their eyes are still in a stage of major growth development that would be disrupted by ocular surgery. Refer to the Instructions for Use and Physician’s Information Brochure for other warnings, precautions, and instructions for the use of the device.